Enthalpy Change of Atomisation
The enthalpy of atomization refers to the amount of change in the heat when the bonds of a compound tend to break and elements of that compound get reduced to singular atoms. For now just read the.

A Level Bond Enthalpy Bond Dissociation Energy Calculations For Enthalpy Of Reaction Ks5 Gce Chemistry Revision Notes
Enthalpy of atomisation ΔHa is the change in enthalpy when one mole of bonds are completely broken to obtain atoms in the gas phase.

. CH 4 g C g 4H g. So enthalpy of atomization of transition metals is very high. Change in enthalpy Δ H Δ U Δ PV Change in enthalpy Δ H Δ U P 2 V 2 P 1 V 1 Change in enthalpy Δ H 30 20 6 Change in enthalpy Δ H 44 L atm.
Enthalpy of atomisation of oxygen. Which equation correctly represents the standard enthalpy change of atomisation of the given. The change of the solid lithium into gaseous state is.
J Enthalpy of atomisation AH is the enthalpy change for the production of one mole of atoms in the gas phase from the element in its standard state. In this case enthalpy of atomization is the same as that of bond dissociation enthalpy. This is often represented by the symbol ΔₐₜH or ΔHₐₜ.
Transition elements have a large number of valence electrons and high effective nuclear charge. It is always endothermic. You will find enthalpy change of atomisation on the Chemguide page lattice enthalpy lattice energy.
Bond dissociation enthalpy is the enthalpy change for a mole of substance to break its covalent. The enthalpy of atomization also atomisation in British english is the enthalpy change that accompanies the total separation of all atoms in a chemical substance either a chemical. Q3 Q4 Q5 Q6 Q7 Q8 Q9.
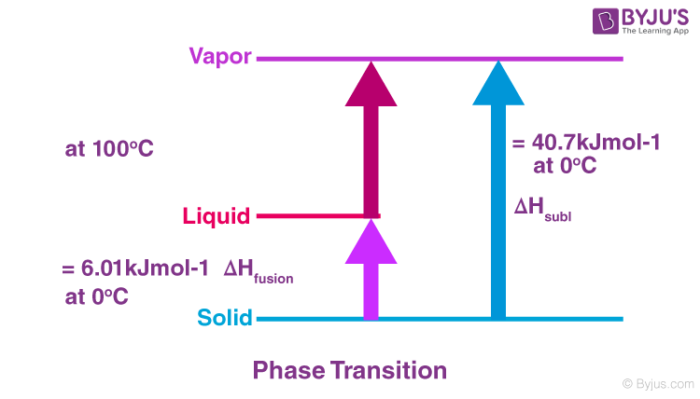
The atomisation enthalpy of a compound is the energy needed to convert 1 mole of a compound into its free gaseous atoms under standard states. The change of the solid lithium into gaseous state is equal to 161kJmol -1 Li s Li g ΔH Ꝋat 161 kJ mol 1 Notice the value of standard enthalpy change of atomisation. The associated standard enthalpy is known as the Standard enthalpy of atomization Δ at H o kJ mol 1 at 29815 K or 25 degrees.
The enthalpy of atomization is the enthalpy change that accompanies the total separation of all atoms in a chemical substance. Is the enthalpy change when 1 mole of gaseous atoms is formed from its elements under standard condition. Statement 2311a defines enthalpy change of atomisation.

Caie 9701 11 O N 20 Q 6 Enthalpy Changes Youtube

For Part I How Do I Write The Equation For Atomisation I Remember In Enthalpy Change Of Atomisation One Mole Of Atom Is Formed From Substance That Is In Its Standard State

Comments
Post a Comment